thalidomide impact on drug testing|thalidomide research : fabrication The wonder of thalidomide, created by West German drugmaker Gruenthal, was its initial claims to be an effective sedative without side effects. But the drug had undergone only minimal testing, and when it was given to . WEBSomente na Abelvolks Store você encontra toda linha de Cordões Oficiais Abelvolks com os melhores preços! Quem Somos; Meus Pedidos; Fale Conosco; Atendimento 6299 972-9966 6299 972-9966 . ABELVOLKS COMERCIO E SERVICOS LTDA - CNPJ: 00.129.393/0001-38. Loja Virtual criada por DLoja Virtual.
{plog:ftitle_list}
Acertar 6 Placares Bet365 - Apuestas Nba Bet365como é um torneio knockout no pokeremagens numeros de roletapedro cantor craptexas hold'em poker 3d deluxe edition pc the pirate bay [O Epoch Times noticiou em 6 de novembro] (repórter da Agência Central de Notícias Guo Chuanxin, Nova Delhi, 6º) Otillo, secretário de Estado adjunto para .
The wonder of thalidomide, created by West German drugmaker Gruenthal, was its initial claims to be an effective sedative without side effects. But the drug had undergone only minimal testing, and when it was given to .
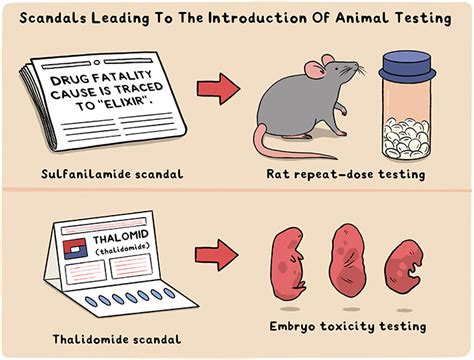
The thalidomide tragedy marked a turning point in toxicity testing, as it prompted United States and international regulatory agencies to develop systematic toxicity testing . Now, drug approval can take between eight and twelve years, involving animal testing and tightly regulated human clinical trials. Despite its harmful side effects, thalidomide is FDA-approved for two uses today—the .In 2006 thalidomide completed its remarkable renaissance becoming the first new agent in over a decade to gain approval for the treatment of plasma cell myeloma. The catastrophic collapse . The catastrophic collapse yet subsequent revival of thalidomide provides important lessons in drug development. Never entirely abandoned by the medical community, thalidomide resurfaced as an important drug once the .
The catastrophic collapse yet subsequent revival of thalidomide provides important lessons in drug development. Never entirely abandoned by the medical community, thalidomide .Of all the drugs developed in the intervening years, thalidomide has undoubtedly had the greatest influence on shaping the Pharmaceutical Industry as we know it today.Strong marketing .
When thalidomide is used for clinical treatment with patient safety programs in place, it is a safe and effective drug [2,21]. Yet, long-term use can cause peripheral neuropathy, and there are . From 1956, scientists tested the drug on healthy adults, patients complaining of anxiety or sleeplessness, nursing mothers, patients with mental illnesses, and children. Studies published in.
It’s fair to say that no other medicine has had more of an effect on the regulatory requirements for safety-testing potential drugs before they go anywhere near a human being. The thalidomide tragedy was responsible for .
It is often claimed that the Food, Drugs, and Cosmetics Act of 1938 prevented a thalidomide tragedy in the USA, in contrast to what occurred in many other countries. 32,40 The approval of thalidomide was, however, not . The drug was not approved in the United States in the 1960s, but as many as 20,000 Americans were given thalidomide in the 1950s and 1960s as part of two clinical trials operated by the American .The first paper describing the pharmacological actions of thalidomide was published in 1956. The drug, then designated as K17, was thought to have sedative effects superior to those of comparator drugs and was thought to be virtually nontoxic. Only 2 years after thalidomide's launch as Contergan in .
Although many of the changes to drug testing and regulation attributed to thalidomide were already underway in the 1950s, new regimes ultimately contributed to greater confidence that thalidomide . With mounting governmental pressures to accelerate the drug approval process, 71 out of 222 drugs placed on the market between 2001 and 2010 came with side effects so serious that the FDA issued black-box warnings (warnings pertaining to potentially serious or lethal side effects of a prescription drug) for these treatments. What is even more . The drug’s potentially harmful effects on the fetuses of certain mammals was not recognized during testing. Teratogenic effects. Thalidomide went on the market as a treatment for morning sickness in more than 40 countries beginning in 1958. It was soon found to have teratogenic effects—producing severe malformations in infants born of . Stock said Grünenthal researchers had conducted all possible tests on thalidomide based on latest science. . that demonstrated thalidomide’s negative side effects. Again, the drug was still .
Although there are some members of the Food and Drug Administration and some members of the medical profession who feel that more extensive and elaborate drug test- ing will prevent another thalidomide episode, the consensus among embryologists and tera- tologists is that absence of malformations in an animal strain is no guarantee that a drug . Thalidomide is a medication used to manage and treat advanced leprosy and multiple myeloma, and various other solid and hematologic malignancies. It is in the immunomodulatory class of medications. This activity reviews the indication, action, and contraindications for thalidomide as a valuable agent in the treatment of leprosy, multiple .Of all the drugs developed in the intervening years, thalidomide has undoubtedly had the greatest influence on shaping the Pharmaceutical Industry as we know it today.Strong marketing pressure in an Industry hungry for new medicines brought an inadequately tested drug to the market, targeted outsourcing quickly expanded the client base and . Introduction Lenalidomide, pomalidomide, and thalidomide are effective treatments for multiple myeloma but are teratogenic. To mitigate this risk, the US Food and Drug Administration (FDA) required risk evaluation and mitigation strategy (REMS) programs for these drugs, which include pregnancy testing among women of childbearing potential—twice .
Structure of thalidomide and its analogs. After removal of thalidomide from the market, an accidental discovery of its immunomodulatory effects was made in erythema nodosum leprosum (ENL) patients in 1965, thus defining a new indication of usage for thalidomide [4, 5].Additional studies and clinical investigations further demonstrated the beneficial effects of thalidomide .Thalidomide comes in capsules, so you can take it at home. Thalidomide is usually taken with other chemotherapy drugs and steroids. Your doctor will decide which combination of drugs you have. Sometimes you may have thalidomide on its own or with just a steroid. You usually have a course of several cycles of treatment over a few months.
Yet, how thalidomide caused its devastating effects in the forming embryo remains unclear. However, studies in the past few years have greatly enhanced our understanding of the molecular mechanisms the drug. This .Thalidomide (α-N-[phthalimido] glutarimide) was first synthesized in 1953 by Ciba, a Swiss pharmaceutical firm, and then in 1954 by Kunz, a chemist at Chemie Grünenthal, a German pharmaceutical company.1,2 On October 1, 1957, Chemie Grünenthal introduced the drug into the market as a sedative.1,3,4 Thalidomide lacked the typical addictive properties of . Although the concept of drug-induced teratogenicity was well established at the time thalidomide was being developed, questions remain today as to whether the effects on embryo-fetal development would have been detected using the standard testing methods. Modern evidentiary standards of drug approval have greatly expanded our ability to predict the effects of drugs, but the interpretation of study data, or the act of distilling raw data into useful information, has become a science in its own right.
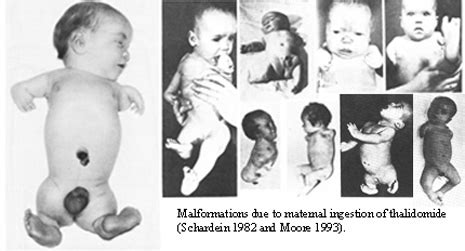
At the time, basic testing was done on the drug, and it was considered not to have any toxic effects on humans. However, unlike today’s level of rigorous testing, the drug was not analyzed for .
thalidomide trials
The current use of animals to test for potential teratogenic effects of drugs and other chemicals dates back to the thalidomide disaster of the late 1950s and early 1960s. Controversy surrounds the following questions: 1. What was known about placental transfer of drugs when thalidomide was developed? 2. Was Another result was that all drugs must undergo formal testing for teratogenic effects; as a result, the teratology field has developed rapidly. Beyond the social and legal implications, other lessons have been learned from the scientific advancements made after Dr Sheskin first used thalidomide in ENL.
Thalidomide had not received regulatory approval in USA, although it had been licensed in Canada, and thus the USA was largely spared the drug's effects. Thalidomide had been introduced into an almost unregulated German market in 1957 as an alternative to barbiturates, widely advertised as being safe for everyone “even during pregnancy for . Thalidomide, a sedative drug first synthesised in 1953, created one of the most dramatic disasters in the history of medicine. From 1958 the drug had been widely praised, advertised, and prescribed on the grounds that it was unusually safe–largely because it was almost impossible to commit suicide with it. Thalidomide was exceptionally effective in, . Kelsey’s wariness was vindicated by revelations of the drug’s devastating effects in pregnancy. Thalidomide precipitated the rapid passage of the 1962 Kefauver-Harris Amendment to the US Food .
Fortunately, drug manufacturer Richardson-Merrell’s NDA for Kevadon—the U.S. trade name for thalidomide—was only the second NDA Kelsey received, meaning she didn’t yet have a backlog of .
The S.T.E.P.S. program, designed by Celgene pharmaceuticals and carried out in pharmacies where thalidomide prescriptions are filled, educates all patients who receive thalidomide about potential risks associated with the drug. Thalidomide has also been associated with a higher occurrence blood clots and nerve and blood disorders.Pregnancy must be excluded in female patients of childbearing potential before starting treatment with thalidomide. A medically supervised pregnancy test should be performed on, or within 3 days prior to, initiation and repeated every 4 weeks thereafter (including 4 weeks after the last dose), except in the case of confirmed tubal sterilisation.

subaru sti compression test
Resultado da Unibet offers online sports betting and casino games online. Enjoy your favorite slots, table games and video poker wherever you are! Sign-up today!
thalidomide impact on drug testing|thalidomide research